Gaertn for The Assessment of Potential Anti HIV Constituents & other Therapeutic Components. (Padmam)
Muhammad Imran
Doctor of Pharmacy
The microscopic cellular analysis and of Nelumo Nicifera Gaertn have established numerous health values of this plant and most recently its Anti HIV components (I)(R)Coclaurine, I(S) NorCocclaurine Quercetin, Glucuronide were isolated from the padma plant leaves and have been identified as anti HIV components. Further studies are to confirm the findings of these research works.
In recent scientific studies of the structure, activity correlations with related alkaloids have confirmed potential Anti-HIV benzylisoquinoline alkaloids and flavonoids from the leaves of Nelumbo nucifera.
In this studies (+)-1(R)-Coclaurine (1) and (−)-1(S)-norcoclaurine (3), together with quercetin 3-O-β-d-glucuronide (4), were isolated from the leaves of Nelumbo nucifera (Nymphaceae), and identified as anti-HIV principles. Compounds 1 and 3 demonstrated potent anti-HIV activity with EC50 values of 0.8 and <0.8 μg/mL, respectively, and therapeutic index (TI) values of >125 and >25, respectively. Compound 4 was less potent (EC50 2 μg/mL).
In a structure–activity relationship study, other benzylisoquinoline, aporphine, and bisbenzylisoquinoline alkaloids, including liensinine (14), negferine (15), and isoliensinine (16), which were previously isolated from the leaves and embryo of Nelumbo nucifera, were evaluated for anti-HIV activity.
Compounds 14–16 showed potent anti-HIV activities with EC50 values of <0.8 μg/mL and TI values of >9.9, >8.6, and >6.5, respectively. Nuciferine (12), an aporphine alkaloid, had an EC50 value of 0.8 μg/mL and TI of 36. In addition, synthetic coclaurine analogs were also evaluated. Compounds 1, 3, 12, and 14–16 can serve as new leads for further development of anti-AIDS agents. See Foot Notes 7-13.
Nelumbo nucifera Gaertn (Family- Nymphaeaceae) is a well known plant in ancient ayurvedic and medical sciences. The objective of this research article is to present to our customers a scientific evaluation of its chemical and medicinal constituents relating to phytochemical, pharmacological microbiological and allied approaches to highlight its heath values. Nelumbo nucifera Gaertn consists of dried flowers of Nelumbo nucifera Gaertn. Syn. Nelumbium speciosum Willd. Fam. Nymphaeaceae; a large, aquatic herb with submerged rhizome, occurring throughout warmer parts of India upto an altitude of 1000 m.

OTHER NAMES
San. – Abja; Aravinda; Pankaja; Utpala
Assam. – Podum
Bengal – Padma phool; Salaphool
Eng. – Lotus
Guj. – Kamal
Hindi – Kamal; Kanwal
Kan. – Kamal; Tavare; Naidile; Tavaregedd
Mal. – Komala; Thamara; Santhamara, Centamarai
Ori. – Padma
Punj. – Kanwal; Pamposh
Tam. – Tamarai; Centamarai
Tel. – Kaluva; Tamarapuvvu
Urdu – Kamal
DESCRIPTION
Microscopy
TS of pedicel shows two layered epidermis consisting of radially elongated cells. Underneath this lies 6 to 8 layered collenchymatous hypodermis embedded with bases of pear shaped air cavities its narrow and being connected with cells of epidermis, the remaining ground tissue is parenchymatous, traversed with conjoint, collateral vascular strands and circular to oval huge air cavities. Small air cavities traverse throughout the phloem tissue of the vascular bundles also simple starch grains, prismatic and cluster crystals of calcium oxalate are present throughout the parenchymatous cells of the ground tissue. (Fig. 1)
Macroscopy
Drug occurs as entire shriveled yellowish brown pieces of flowers, 10 to 15 cm in diameter, comprising of calyx, corolla, androecium, gynoecium, thalamus and pedicel; sepals leaf-like, 3 to 5 cm long, 1.3 to 2 cm wide, dark brown, broken; petals numerous, elliptic, obtuse, membranous, finely veined, 2 to 4 cm long, 1.2 to 2 cm wide, yellowish-brown; anther, erect, linear 1.4 to 2 cm long, extended into clavate appendages; gynoecium apocarpous; carpels many, free, embedded in a creamy, topped fleshy thalamus (torus) 3-5 cm long and 2.5 to 3 cm wide; style minute, stigma terminal, ovary single celled, ovule solitary, suspended, anatropus. (Plate-1)
The transverse section of sepal is broad centrally and has thin narrow margins. The cells of lower and upper epidermis are papillate. In surface view these are straight walled and angular, the anomocytic stomata are present on both the surfaces. On upper side twin stomata are also observed. The single layered hypodermis is followed by loosely arranged mesophyll cells. Number of collateral vascular bundles are scattered in mesophyll region. Starch grains and large air cavities are present in mesophyll region and phloem parenchyma, margin of sepals consists of 1-2 layers of loosely arranged parenchymatous cells. (Fig.2).
TS of petals are almost similar to sepals except the cells of upper and lower epidermis are highly papillate and thick walled, stomata are present on both the surfaces and are sunken and anomocytic type,cluster crystal of calcium oxalate are present in epidermal cells. (Fig. 2).
Androecium – A majority of the stamens have clavate shaped distal sterile appendages with obtuse apex. Four slender long, sublateral, microsporangia occur in pairs. A transverse section of the filament and appandage appears almost circular in outline covered by single layered papillate epidermis followed by single layered hypodermis which is filled with reddish brown contents, followed by loosely arranged parenchymatous mesophyll. The vascular supply varying from 1-5 bundles. In the basal region large central bundle flanked by 4 lateral bundles. (Fig. 3).
TS passing through microsporangia shows a single layered epidermis followed by a radially elongated endothecial layer followed by 4-5 celled parenchymatous cells. The vascular supply is reduced to single bundle. (Fig. 3)
Pollen grains – Pollen grains are 2-4 zonocolporate, size 72 x 66 mm (55.40 – 72 X 44.32 – 62 mm) in diameter, exine thicker than nexine, tectate – punctate. (Fig. 3)
Powder – Powder light brown in colour, sweet in taste with no characteristic odour, on microscopical examination it shows rosette and prismatic crystals of calcium oxalate, pieces of papillate epidermis, aircavities, pollengrain, vessels, epidermal cells with stomata of petals and sepals. (Fig.4).
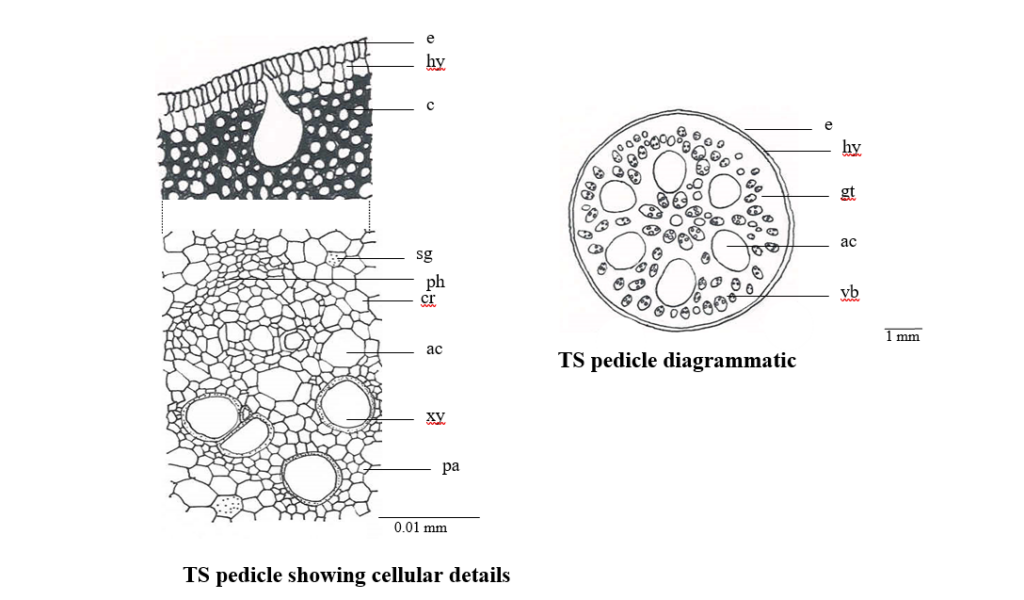
Figure 1. Microscopy of Nelumbo nucifera flower (pedicle)Abbreviations: ap, appendage; c, collenchyma; cr, crystal; e, epidermis; gt, ground tissue; hy, hypodermis; pa, parenchyma; ps, pollen sac; sg, starch grain; xy, xylem vb, vascular bundle.
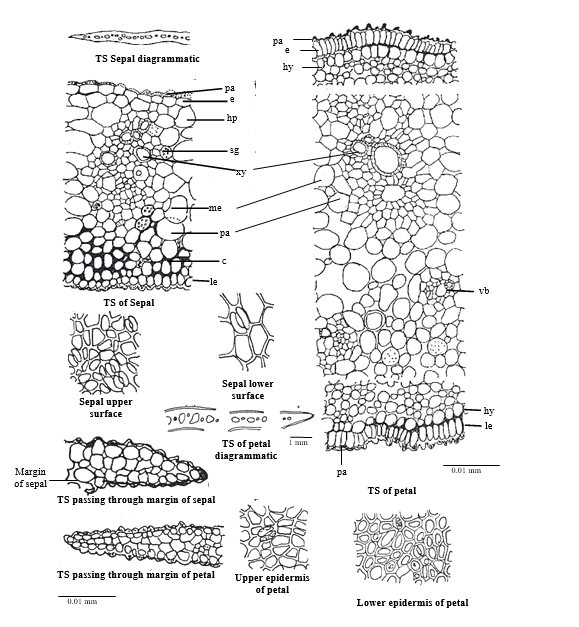
Figure 2. Microscopy of Nelumbo nucifera flower (sepals and petals)
Abbreviations: c, collenchyma; e, epidermis; hy, hypodermis; le, lower epidermis; me, mesophyll; pa, parenchyma; sg, starch grain; vb, vascular bundle; xy, xylem;
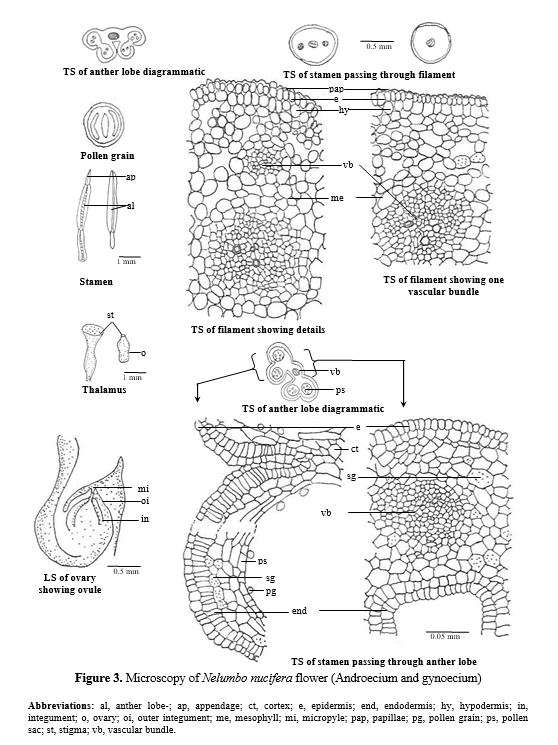

Figure 4. Powder study of Nelumbo nucifera flower
Abbreviations: (Powder- A, pollen grain; B, cluster crystal; C, stomata of petal; D&E, epidermal cells and stomata of sepal; F, crystal and epidermal cells of petal; G, air cavity; H, papillate epidermis); cr, crystals.
CHEMICAL CONSTITUENTS
Major
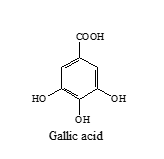
Gallic acid (No supporting literature available till date)
Others
Linalool2, palmitic acid2, 1,4-dimethoxybenzene2. myristic acid2, b-pinene2, limonene2, trans-ocimenene2, g-terpinene2, b-caryophyllene2, a-humulene2, naphthalene2, methyl naphthalene2, dimethyl naphthalene2, n-tetradecane2, n-pentadecane2, n-hexadecane2, n-heptadecane2, n-octadecane2, n-nonadecane2, n-eicosane2, n-heneicosane2, n-docosane2, n-tricosane2, n-tetracosane2, n-pentacosane2, n-hexacosane2, n-heptacosane2, n-octacosane2, n-nonacosane2, n-triacontane2, n-hentriacontane2, n-dotriacontane2, n-tritriacontane2, n-hexadecane2, n-nonadecane2, n-eicosane2, n-tricosane2, 1,8-cineole2, caryophyllene oxide2, d-cadinol2, terpinen-4-ol2, a-terpineol2, geranyl linalool2, phytol2, methyl propyl phenol2, cis–jasmone2, methyl palmitate2, methyl stearate2, methyl eicosanoate2, methyl tricosanoate2, ethyl hexanoate2, ethyl stearate2, linalyl hexanoate2.
TLC IDENTITY TEST
Test solution:
Reflux 5 g of the powdered drug with methanol on a water bath for 30 min. Filter and remove the solvent under reduced pressure consecutively three times, dissolve 25 mg of the residue in 10 ml of methanol. Use this solution for TLC profile.
Standard solution:
Dissolve 10 mg of gallic acid in 10 ml of methanol.
Solvent system:
Toluene: Ethyl acetate: Formic acid (5:5:1)
Procedure:
Apply 10 ml each of the test and standard solutions on a precoated silica gel 60 F254 TLC plate (E. Merck) of uniform thickness 0.2 mm. Develop the plate in the solvent system in twin trough chamber to a distance of 8 cm.
Visualization:
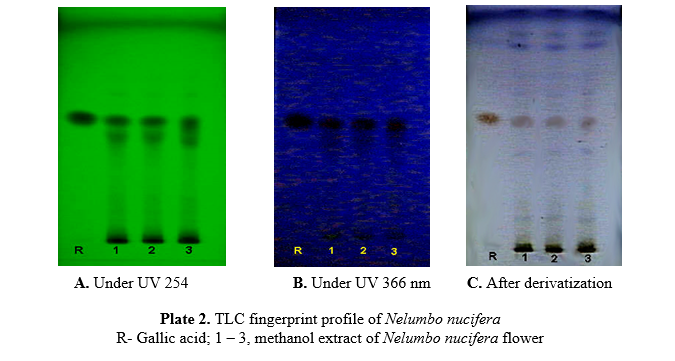
Observe the developed plate under UV light at 254 (Plate 2A) and 366nm (Plate 2B).and under visible light after derivatization with anisaldehyde-sulfuric acid reagent and heat the plate at 110°C for 10 min. (Plate 2C). Record the Rf values and colour of the resolved bands (Table 1).
Evaluation:
A band (Rf= 0.55) corresponding to gallic acid is visible in both reference solution and test solution tracks.
Table 1. TLC pattern of test solution of Nelumbo nucifera methanol extract.
Table 1. TLC pattern of test solution of Nelumbo nucifera methanol extract.| At UV 254 nm | At UV 366 nm | Under Visible | Afterb Derivatization | ||
|---|---|---|---|---|---|
| Rfbvalue | Colour of the band | Rf value | Colour of the band | Rf value | Colour of the band |
| 0.14 | Black | – | – | 0.46 | Light Brown |
| 0.34 | Black | – | – | 0.55 (Gallic acid) | Light Brown |
| 0.46 | Black | 0.46 | Light blak | 0.83 | Violet |
| 0.55 (Gallic acid) | Black | 0.55 (Gallic acid) | Black | 0.96 | Light Brown |
ASSAY / ANALYTICAL METHOD
TLC densitometric estimation of gallic acid.
TLC plates
Precoated silica gel 60 F254 (E. Merck) of 0.2 mm thickness.
Solvent system
Toluene: Ethyl acetate: Formic acid (5: 5: 1)
Scanning
Standard solution:
Dissolve 10 mg of gallic acid in 10 ml of methanol from this stock solution prepare standard solutions of 20-100 mg/ml by transferring aliquots (0.2 to 1 ml) of stock solution to 10 ml volumetric flask and adjusting the volume to 10 ml with methanol.
Calibration curve:
Apply 10 ml of each of the standard solutions (200-1000 ng per spot) on a precoated silica gel 60 F254 TLC plate. Develop the plate in the solvent system in twin trough chamber to a distance of 8 cm, dry it and scan densitometrically at 278 nm, record the peak area and prepare the calibration curve by plotting peak area against concentration of the gallic acid applied.
Estimation of gallic acid in the drug:
Apply 10 ml of the test and standard solutions in triplicate on a precoated TLC plate (E. Merck) of uniform thickness of 0.2 mm. Develop the plate in the solvent system and record the peak area of gallic acid as described above for the calibration curve. Calculate the amount of gallic acid present in the sample from calibration curve of gallic acid. (Fig.2).
The percentage of gallic acid ranges from 0.85 to 0.95 (w/w) in the samples analysed.
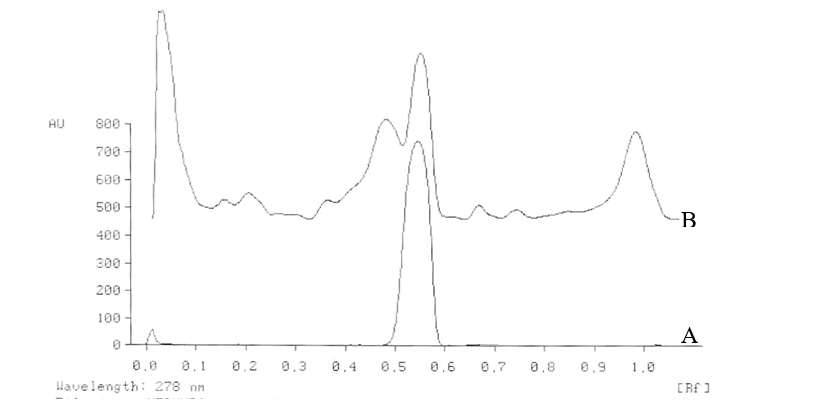
Figure 2. TLC densitometric scan at 278 nm of test solution of methanolic extract of Nelumbo nucifera flowers. A. Gallic acid standard B. Test solution
QUANTITATIVE STANDARDS
- Foreign matter: Not more than 2 per cent.
- Moisture content: Not more than 5 per cent.
- Total ash: Not more than 6 per cent.
- Acid-insoluble ash: Not more than 1 per cent.
- Ethanol-soluble extractive: Not less than 16 per cent.
- Water-soluble extractive: Not less than 30 per cent.
ADULTERANTS/ SUBSTITUTES
The petals and whole flowers of Nymphaea species can be adulterated or substituted, the petals and sepals of Nelumbo nucifera can be differentiated by the absence of sclerieds.
Pharmacology
The aqueous and alcoholic extracts of the flowers showed anti-hypoglycemic activity in rabbits3 . On chronic treatment there was no sustained suppression of fasting blood sugar level4. The ethanolic extract of the seeds reported to have significant antioxidant and hepatoprtective activities5.
THERAPEUTIC category
Haemorrhagic disorders6.
Safety aspects
Drug is traditionally considered safe with in prescribed dose6.
Dosage
12-24 g of the drug for decoction6.
ReferenceS
- Mehrotra S, Shome U, Sharma, HP. Pharmacognostic studies on the flower of Nelumbo nucifera Gaertn. New Botanist 2017; 14: 126-146.
- Omata A, Yomogida K, Nakamura S,Ohta T, Izawa Y, Watanabe S. The scent of lotus flowers. J Ess Oil Res 1991; 3:221-227.
- Huralikuppi JC, Christopher AB, Stephen PM. Anti-diabetic effect of Nelumbo nucifera (Gaertn): Part I preliminary studies in rabbits. Phytothera Res 1991; 5 54-58.
- Huralikuppi JC, Cristopher AP, Stephen PM. Antidiabetic effect of Nilumbo nucifera Gaertn part II. Phytother Res 1991; 5: 217-123.
- Sohn DH, Kim YC, Oh SH, Park EJ, Li X, Lee BH. Hepatoprotective and free radical scavenging effects of Nelumbo nucifera. Phytomed 2003; 10: 165-169.
- The Ayurvedic Pharmacopoeia of India. Part-I Vol II, 1st Edition, Govt. of India. Ministry of Health and Family Welfare, Department of ISM&H, New Delhi 1999. p. 69-70.
- Faculty of Pharmaceutical Sciences, Niigata University of Pharmacy and Applied Life Sciences, Niigata 950-2081, Japan
- Brion Research Institute of Taiwan, Taipei, Taiwan 100, Republic of China
- Faculty of Pharmacy, Meijo University, Tempaku-ku, Nagoya468, Japan
- Tokai Gakuen University, Miyoshi, Aichi 470-0207, Japan
- Faculty of Pharmaceutical Sciences, Fukuoka University, Fukuoka 814-0180, Japan
- BBI-Biotech Research Laboratories, Perry Parkway, Gaithersburg, MD 2087, USA
- Natural Products Laboratory, School of Pharmacy, University of North Carolina, Chapel Hill, NC 27599, USA
